21
Therefore none of the protons in the methyl group has specific position with respect to the remainder of the molecule, and there is a huge number of possible conformations for the acetaldehyde:
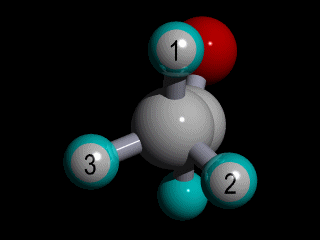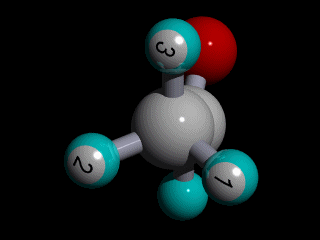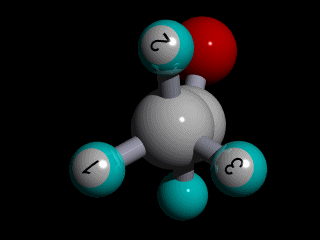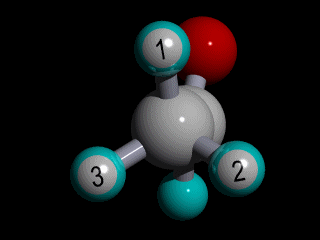
The rotation of the methyl group is so fast compared to the time scale of an NMR experiment that the methyl protons experience an electronic shielding that is the average over time of the electronic distribution of all the the possible conformations of the remainder of the molecule.
This is the case for all the methyl protons ; all have the same average chemical shift.
Since the rate of rotation around single bonds is temperature-dependent, the number of signals is also dependent on the temperature.
Want to know more?
Otherwise continue here!