131
Temperature dependence of the signal number within flexible systems
The methyl group within acetaldehyd CH3-CHO is able to rotate around the single bond between the two carbon atoms. The result of this rotation is an averaged identical chemical shift for all methyl hydrogens. Hence we observe one signal only.The situation changes with an rotational speed slowed down due to low temperature. In this case the most favoured conformations become "freezed". The most favoured conformation in the case of acetaldehyd are I, V and IX. In all three conformations exist two different chemical environments for the hydrogens atoms.
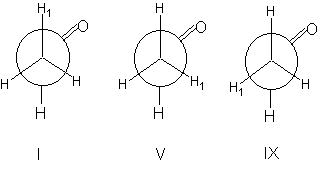
We would observe two signals with an integral ratio of 1 : 2 (see step 26). In somewhat more complex systems such measurements at low temperatures enable possibilities to investigate conformations and conformational equilibria.
And now back to somewhat easier systems