9
|
Your answer was wrong!
Of course, every 1H atom in a compound will
contribute a resonance signal. But consider the following example:
1,1-Dibromomethene contains two protons. It shows, however, only one
resonance line!
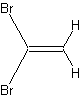
The reason for this will become quite obvious if you have another look
at the second item on page 8.
Fill in the blanks in the
following text:
The two protons have the  electronic
enironment, that is, their spatial relationships to the other atoms in
the molecule are electronic
enironment, that is, their spatial relationships to the other atoms in
the molecule are  , and the character
of the neighboring chemical bonds is , and the character
of the neighboring chemical bonds is  for both protons.
Therefore they have the for both protons.
Therefore they have the  chemical shifts,
and their signals appear at chemical shifts,
and their signals appear at  positions in the
spectrum. positions in the
spectrum.
Now go back to step 8
and reconsider your answer! |
|
|
