7
|
Compare the results of
your own theoretical considerations with the following solutions:
-
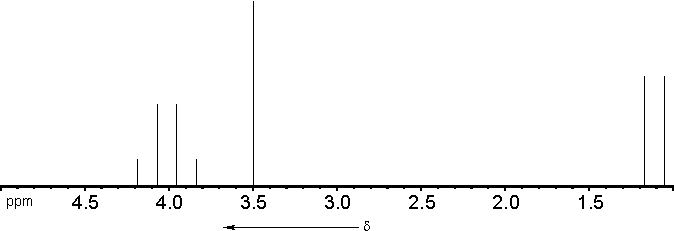
1. Number of resonance signals = 3
- 1 signal for the -OCH3-
protons
1 signal for the -CH< - proton
1 signal for the -CH3 - protons
Wrong answer? Click here! |
-
2. Chemical shift of the resonance signals:
- -OCH3 :
d = 3,3 .. 4,1 ppm
-CH(OR)2 : d
= 2,6 .. 5,3 ppm
-CH3 :
d = 0,6
.. 1,9 ppm
Wrong answer? Click here! |
-
3. Relative intensities of the resonance
signals:
-
| -OCH3 |
: 6 |
| -CH< |
: 1 |
| -CH3 |
: 3 |
Wrong answer? Click here! |
-
4. Line splitting due to spin-spin couplings:
-
| -OCH3 |
: singulet |
| -CH< |
: quartet ( Intensities
1/8:3/8:3/8:1/8) |
| -CH3 |
: doublet ( Intensities 3/2:3/2 ) |
Wrong answer? Click here! |
-
5. Coupling constant
- JCH - CH3
» 7Hz
Wrong answer? Click here! |
The theoretical spectrum should therefore look like
this:
| Now compare
your line spectrum again with the actual spectrum |
|
