63
| Substance | Equivalent protons | Relative intensities | |
|---|---|---|---|
| A) | HCºC-CH3 | a) HCº | 1 |
| b) -CH3 | 3 | ||
| B) | 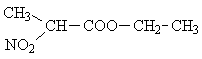 |
a) CH3-CH< | 3 |
| b) >CH- | 1 | ||
| c) -CH2- | 2 | ||
| d) -CH3 | 3 | ||
| C) | H2C=C=CHBr | a)H2C= | 2 |
| b) =CHBr | 1 | ||
| D) | (CH3)2CHOCH(CH3)2 | a) 2 * (CH3)2C< | 6 (12) |
| b) 2 * >CH-O | 1 (2) | ||
| E) | 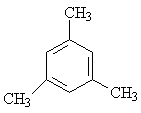 |
a) 3 * -CH3 | 3 (9) |
| b) 3 * Phenyl-H | 1 (3) |
If you made mistakes in identifying groups of equivalent protons, you might want to re-read the unit on page 8.
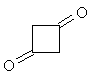
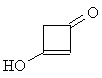
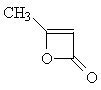
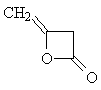
| Now apply what you have learned about the shape
of 1H-NMR spectra to solve the next problem: Two molecules of ketene CH2=C=O react spontaneously to form the dimeric diketene C4H4O2. There are two signals in the 1H-NMR spectrum 2 of diketene. The ratio of their relative intensities is 1:1. Considering this, which of the following four structures is the correct one for the diketene? Once you have come up with a good reason for your choice, click on the image of the correct isomer to see whether you were right!
Would you like to get a hint? |