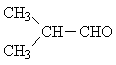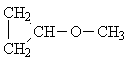48
Answers:
| Molecule | Number of signals |
Proton groups |
Expected chemical shift d [ppm] |
|
|---|---|---|---|---|
| A) | CH3-CO-CH3 | 1 | CH3-CO | 1,7 ... 2,7 |
| B) | Br2=CH2 | 1 | >C=CH3 | 4,3 ... 5,3 |
| C) | (CH3)2-CH-O-CH(CH3)2 | 2 | -CH3 | 0,6 ... 1,9 |
| >CH-O- | 2,5 ... 5,3 | |||
| D) | CH3-CH2-CHO | 3 | -CH3 | 0,6 ... 1,9 |
| -CH2-CO | 1,9 ... 3,2 | |||
| -CHO | 9,1 ... 10 | |||
| E) |  |
2 | C6H3-CH3 | 2,0 ... 2,8 |
| C6H3-(CH3)3 | 2,5 ... 5,3 |
Did you have a problem with the number of signals expected? Go back to page 8 before you continue!
Before you move on to the next set of problems, you should try to do one more problem. This one is a bit more complex and will give you a chance to apply what you have learned about the number of lines in a spectrum and their expected chemical shifts in the previous excercises.