19
Here is an example: All atoms of p-bromo-nitrobenzene are in one plane.
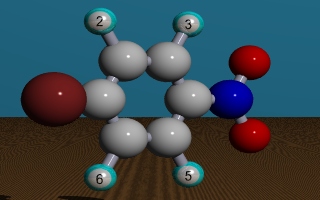
There are now two possible approaches to identify equivalent protons:
a) Using a specific proton as a reference point, characterize the remainder of the molecule. In the case of H-2, for example, the other substituents are:
- With H-2 as the reference point:
- ortho-substituents at the benzene ring: Br- and H-3
- meta-substituents at the benzene ring: H-6 and NO2-
- para-substituents at the benzene ring: H-5
Now you can compare this set of relationships with the ones for each of the of the other protons.
- With H-6 as a reference point:
- ortho-substituents at the benzene ring: H-5 and Br-
- meta-substituents at the benzene ring: NO2- and H-2
- para-substituents at the benzene ring: H-3
H-2 and H-6 show similar relationships to the substitutents, which means that they are equivalent
b) Look for symmetry elements that define the molecule, such as mirror symmetries, rotational symmetries or combinations of the two.
As a case in point, the molecule in this example has a two-fold rotational symmetry with a rotational axis C2 through the Br and N atoms:
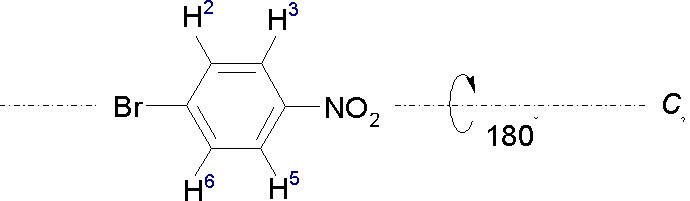
Through a l80° rotation around this axis
Now go back and determine the number of
equivalent protons and the number of NMR signals in the following
molecules:
|





