122
Answers:
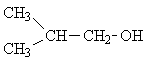
Reasoning:
In this example we will try to find the correct answer by eliminating all the false ones. To do so we will compare the spectra of all possible isomers with the actual spectrum of our mystery molecule.| Isomer | Signals | Groups | d [ppm] | Irel. | Multiplicity | |
|---|---|---|---|---|---|---|
| 1. | CH3-CH2-CH2-CH2-OH | 5 | ||||
| 2. | 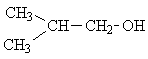 |
4 | (CH3)2
>CH- CH2 -OH |
» 1
»1.8 »3.6 up to 5.2 |
6 1 2 1 |
2 21(7*3) 4(2*2) 3 |
| 3. | 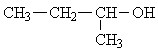 |
5 | ||||
| 4. | 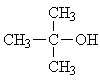 |
2 | ||||
| 5. | CH3-CH2-O-CH2-CH3 | 2 | ||||
| 6. | CH3-O-CH2-CH2-CH3 | 4 | CH3 CH2 CH3-O- CH2-O- |
»1 »1.5 »3.6 »3.8 |
3 2 3 2 |
3 12(3*4) 1 3 |
| 7. | 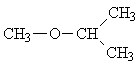 |
3 |
The spectra of only two of the seven possible isomers have the same number of lines as the experimental spectrum. Picking isomer 2 of these two options is the rather obvious solution, once you consider the relative intensities and the respective multiplicities of the signals.
Now on to one last problem of this type!