112
Answer: |
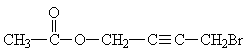 |
Reasoning:
Since molecule c) can be eliminated solely based on the number of signals in the spectrum, we only have to decide between options a) and b).The long-range coupling between the CH2-groups in both molecules a) and b) gives rise in either case to two triplets.
The only difference that we can predict lies in the characteristic expectation ranges for the spectral postions of the CH3- and CH2-groups, respectively:
| Molecule a) | Molecule b) | |
|---|---|---|
| CH3-group | CH3 - CO - | CH3 - O - |
| CH2-Gruppe | - CH2 - O - | - CH2 - CO - |
Evidently option a) is the correct answer!
| Molecule | Equivalent protons | Number of signals | d [ppm] (expected) | d [ppm] (observed) | Multiplizität (expected) | Multiplizität (observed) |
|---|---|---|---|---|---|---|
| CH3-CO-O-CH2-CºC-CH2Br | CH3
- CO - - O - CH2 - - CH2Br |
3 | 1.7 ... 2.7
3.3 ... 4.5 2.3 ... 4.7 |
2.12
4.3 4.7 |
1 3 3 |
1 3 3 |
| CH3-O-CO-CH2-CºC-CH2Br | CH3
- O - - CO - CH2 - - CH2Br |
3 | 3.3 ... 4.1
1.9 ... 3.2 2.3 ...4.7 |
2.12 4.3 4.7 |
1 3 3 |
1 3 3 |
| CH3-CO-O-CH2-CH=C=CHBr | CH3
- CO - - O - CH2 - - CH = = CHBr |
4 |
If you ran into trouble while trying to solve this problem, then you might want to go back and review the section on chemical shifts one more time!
Otherwise move on to the next problem!