111
Answer: |
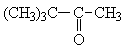 |
Reasoning:
This question can already be decided by simply counting the number of resonance signals. Only the second molecule would give rise to two signals in the spectrum.| Molecule | number of groups of equivalent protons | Number of signals (expected) | Signals (observed) |
|---|---|---|---|
|
|
a) - C(CH3)3 b) - CH2 - c) - CHO |
|
|
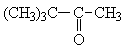 |
a) - C(CH3)3 b) - CO - CH3 |
|
|
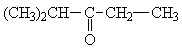 |
a) - CH2 - CH3 b) - CH(CH3)2 c) - CO - CH2 - d) > CH - CO - |
|
|
If you had troubles with this problem, you might want to go back and have another look at the section about the number of resonance signals.
If you came up with the correct answer for this problem, then move on to the next one on page 110a!