106
|
Oops, wrong answer!
You ought to know better by now! (Didn't your mother teach you better?)
| If you compare the two
molecules |
| A) |
|
A |
|
X |
|
|
| CH3 |
- |
CH |
- |
COOD |
|
|
| |
|
|
|
|
ND2 |
|
|
|
|
and |
| B) |
|
|
|
A |
|
X |
| D2N |
- |
CH2 |
- |
CH2COOD |
|
|
|
you will come to the following conclusions:
- Both molecules show in their NMR spectra
 signals, since their
proton groups are signals, since their
proton groups are 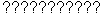 . .
- The proton groups in both molecules couple with each
other because in both cases the respective groups are
 . .
- The two NMR signals of the molecule B)
would be split due to nA = 2 and nX = 2 both into
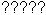 . .
The molecule A) would show in its spectrum a
doublet and a quartet, because nX =  and nA = and nA = 
Therefore the observed spectrum can only be caused by molecule A)!
In addition to the signals from its aromatic
protons in its 1H-NMR spectrum, a substituted
benzene derivative of the composition C9H12
gives rise to
- a doublet (1 : 1) and
- a heptet (1 : 6 : 15 : 20 : 15 : 6 : 1).
Can you propose a structure that is in agreement with these data? Check
here for the
solution! |
|
 signals, since their
proton groups are
signals, since their
proton groups are 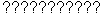 .
. .
.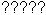 .
.  and nA =
and nA = 